Redefining humanly possible through the potential of muscle cell technology
Cook MyoSite is dedicated to commercializing muscle cell therapies and advancing the field of muscle cell technologies.


The most trusted name in muscle cell technology
Cook MyoSite was founded in 2002 to make regenerative medicine a part of everyday medicine. Since then, we’ve been working tirelessly to bring first-in-kind cell technologies to market with the most rigorous quality standards.
This dedication to quality extends to our Laboratory Research Services portfolio, manufactured under an ISO9001:2015 Certified Quality Management System.


Regenerative medicine can be everyday medicine
The Cook MyoSite mission began with a simple belief that the human body is capable of wonderful restorative potential. The investigational iltamiocel platform harnesses this potential by targeting the causes of muscle dysfunction, not just the symptoms.
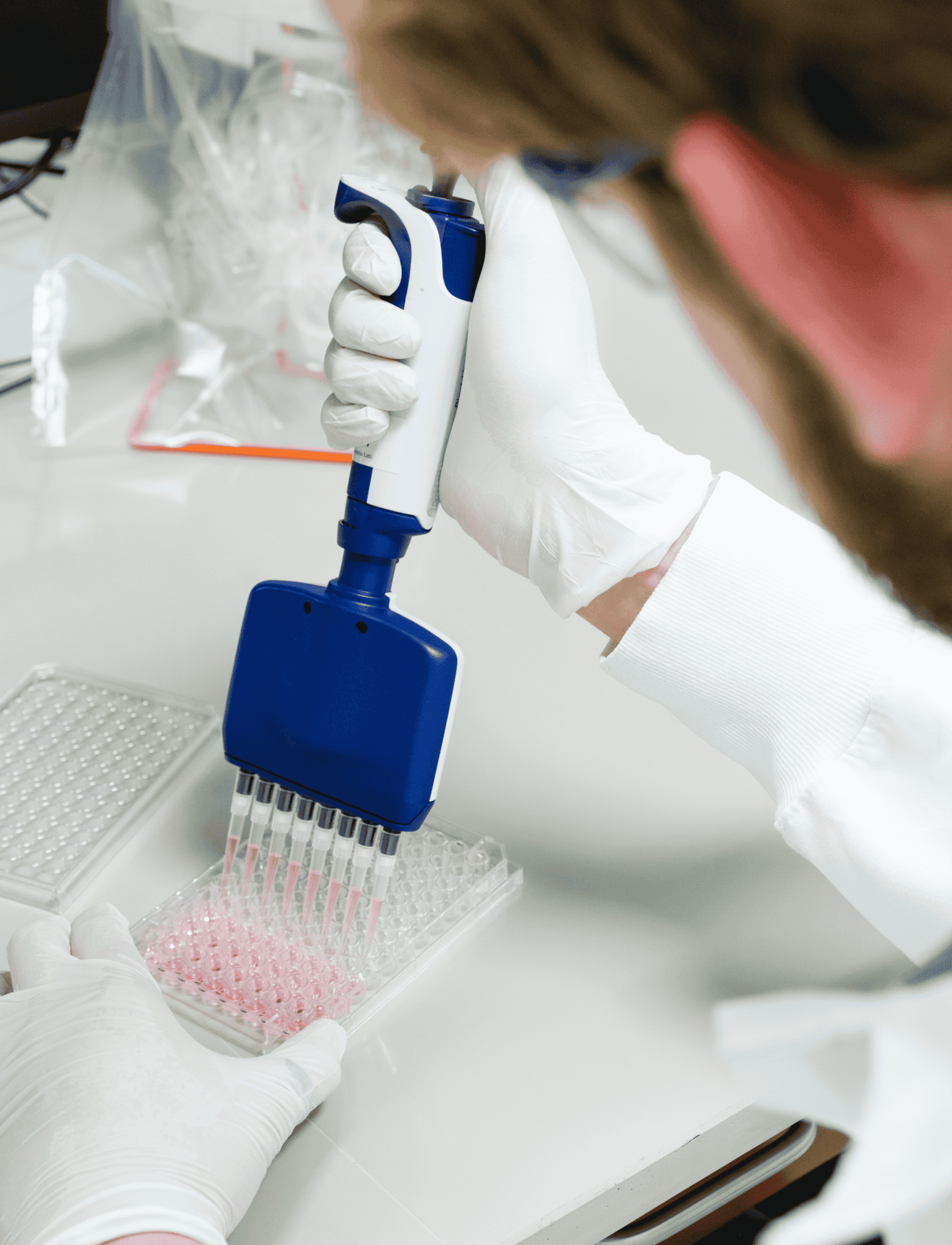


Add some muscle to your research
Over 20 years of clinical development, Cook MyoSite has gained deep insight into muscle cell manufacturing, analysis, and culture. We offer the most reliable and highly characterized primary human skeletal muscle cells on the market, along with custom services to meet your particular research needs.
Our cells are manufactured under highly controlled conditions and cataloged to ensure future availability. Our cells are optimized for use with MyoTonic media products and come with expert support from Cook MyoSite research scientists.
CLINICAL RESEARCH SPOTLIGHT
DigniFI Fecal Incontinence Study: First Participant Treated
Cook MyoSite announced that the first participant was treated in the DigniFI Study, a two-stage, randomized, controlled Phase III trial comparing the safety and efficacy of iltamiocel with placebo in the treatment of female participants with chronic fecal incontinence and a history of obstetric anal sphincter injury (OASI). The procedure was performed on August 19 at Segal Trials in Miami, FL, by Principal Investigator Steven E. Chavoustie, MD, FACOG, CCRP.